Dieldrin contamination and processes leading to its degradation
Learn more about one of the POPs called dieldrin. It is one of the most persistent of the lot.
Most of the processes described for dieldrin are more or less relevant for all other chlorinated persistent pollutants listed under the Stockholm convention. The behaviour of these type of compounds (i.e. organochlorines) in our environment is overall very similar.
The following is a review which I submitted as part of my honours year during my undergrad studies. It received the Frank Gibbons award which was awarded by Soil Science Australia for overall effectiveness in communicating research to a scientific audience.
Introduction
Dieldrin is a toxic and persistent organochlorine chemical and a broad spectrum insecticide which was internationally listed as a persistent organic pollutant (POP) and banned by the Stockholm convention in 20011. In Victoria, dieldrin together with DDT (another POP) was widely used to control a range of insect pests in different crops such as tobacco, potatoes, grains, fruits and pastures. Dieldrin started to be phased out from the 1960’s but remained effectively in use, before it was finally prohibited in Victoria for any agricultural use in 1987. Due to its chemical nature dieldrin is one of the most stable organochlorine chemicals and persisted in top soils (0 - 10 cm) until today, 30 years after its last known use, and therefore caused widespread contamination of agricultural enterprises in Victoria2. Especially beef producers and diaries were affected because animals that later grazed on contaminated pastures continued to consume and accumulate dieldrin by way of soil particles that inadvertently enter the animal while grazing, with the result that dieldrin accumulated in fat tissue and meat products3. It followed that Australian beef exports were threatened when dieldrin concentrations in meat exceeded minimum residue levels after 1987 with wide ranging consequences for the industry until today, involving the set-up of a national organochlorine residue management (NORM) program2 to monitor meat quality and manage contaminated properties. Soil dieldrin concentrations above 0.06 ppm in pasture soils are considered too high for continuous grazing because the meat of grazing animals would accumulate dieldrin above minimum residue levels and become unsellable2. However concentrations of dieldrin in Victorian topsoils remain exceptionally high even compared to sites around the globe4 and can exceed 2 ppm. Hence it continues to dampen land and produce prices, and overall productivity of affected Victorian agricultural areas.
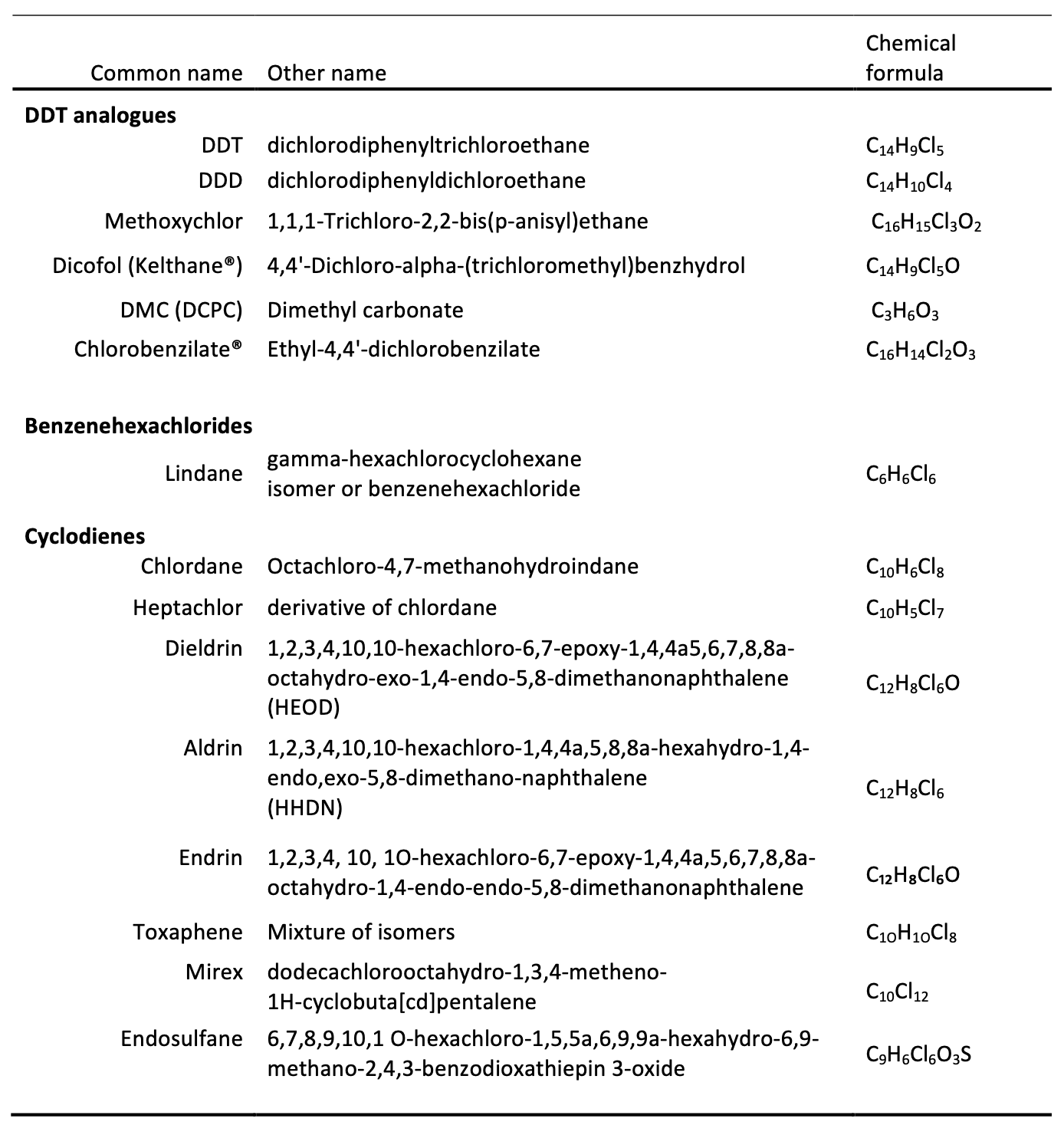
There are various processes leading to dieldrin-disappearance in soils and the rate of these processes depend on different, interrelated environmental factors. The scope of this assay is therefore to provide insights to what is currently known about processes relating to persistence and breakdown of dieldrin in soils. The chemical characteristics of dieldrin, the general processes that determine its fate in soils and the abiotic and biotic factors that affect the breakdown of dieldrin in soils are discussed. Knowledge gaps and the potential for further research are addressed in the conclusion.
Dieldrin chemical characteristics
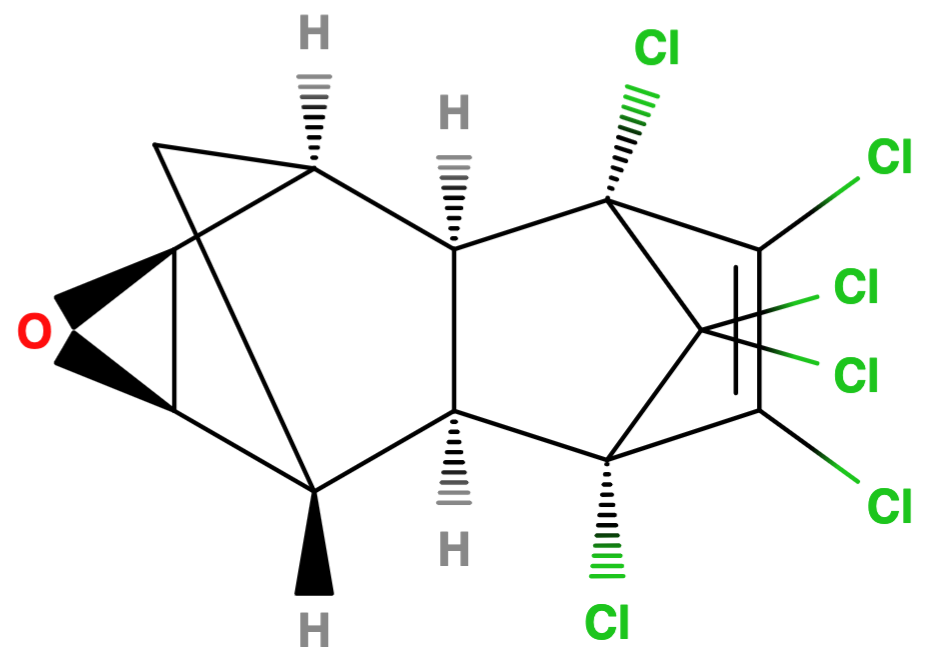
Products sold as dieldrin contained at least 85% of 1,2,3,4,10,10-hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-exo-1,4-endo-5,8-dimethanonaphthalene (HEOD) and was used as a non-systemic broad spectrum insecticide. It is a lipophilic, synthetic organic compound in the chemical group of chlorinated hydrocarbons that was sold as wettable powders, emulsifiable concentrates, dusts, granules or solutions and was manufactured by Shell International Chemical Co. Ltd.
Chlorinated hydrobarbons are categorised into (1) DDT derivatives, (2) benzene hexachlorides (BHC) and (3) cyclodienes - see below Table with examples5. Dieldrin is a cyclodiene compound and the epoxy of the non-toxic aldrin5 consisting of an epoxide ring and the chlorinated carbons. It appears to be unusually stable and apolar despite the strained epoxide ring which makes chemical species usually more polar and reactive. Pure dieldrin has a melting point of 177˚C and is almost insoluble in water (at 0.25 mg L-1)6. It is slightly soluble in petroleum oils, moderately soluble in acetone and soluble in aromatic solvents7. Moreover, it is relatively stable to alkali, weak acid and light, and has a similar volatility to DDT8.
Processes that determine the fate of dieldrin in soils
Transport
Any pesticide applied to soil undergoes transport, retention and transformation processes which are interrelated and affected by climate, vegetation and soil properties. Dieldrin does volatilise into its gaseous phase and can be transported long distances with the atmosphere9. In fact numerous organochlorines including dieldrin have been measured as far as in the Arctic10. Leaching of dieldrin down the soil profile was considered to be insignificant due to its insolubility in water11. Hence it seemed unlikely to leach into underlying groundwater resources but rather pollute other water courses through run-off erosion. Nonetheless, recently dieldrin and other organochlorines were found up to 1.2 m down a profile of a Vertisol of the Namoi valley in New South Wales, Australia12. Weaver et al. (2012) concluded that it was possible that the organochlorines could in fact move further down the profile and indeed pollute groundwater. This was also concluded after an earlier controlled field trial in the Netherlands where it was detected at 50-60 cm depths 13 after 20 years of application. Hence it is hypothesised that if adsorbed to suspended soil colloids dieldrin could ‘hitchhike’ down the soil profile.
Retention
Retention (or adsorption) is a reversible or irreversible chemical process that is determined by the bonding characteristics between the insecticide, clay minerals and organic matter and ultimately determines the bioavailability of dieldrin in soil solution14. Given the persistence of dieldrin in soils, retention processes likely dominate over other processes1516. Clay minerals17 and organic matter18 are known adsorbents for pesticides and these processes are explained in detail by Koskinen and Harper (1990)19. For many insecticides including lindane and probably also dieldrin this is an exothermic process (i.e. they are more soluble at higher soil temperatures)20.
However, bonding mechanisms of dieldrin to soil colloids are not yet understood and in fact it would be unusual for a non-polar and non-ionic chemical species such as dieldrin to react and bond with negatively charged mineral surfaces. One possible mechanism was described by Green (1974)21 whereby non-polar pesticides could be polarised by electrostatic forces and subsequently bond to clay surfaces through hydrogen bridges. Alternatively, van der Waals forces were proposed to be the principle force of attraction between clays and non-polar and non-ionic pesticides.
Furthermore, a low pH and high electrolyte concentration of the soil water both decrease solubility and therefore increase affinity of pesticides to clays21. It was also reported that the bioavailability of dieldrin in soil decreases over time1822 - possibly due to continuing adsorption reactions of the pesticides to minerals or organic matter over time.
Biochemical Processes
Plants and microorganisms determine the biological processes involving POPs in soil. It is understood that plant roots can take up various synthetic organic compounds but that the efficiency of uptake is higher with compounds of higher water solubility (Sicbaldi et al. 1997). Given that dieldrin is nearly insoluble in water, other processes must occur for efficient plant uptake. For example, strongly hydrophobic molecules such as dieldrin can penetrate and translocate through plasma membranes of roots more easily23 or roots may release acidic exudates that desorb sequestered POPs2425. Recently, it also became clear that the role of plant-microbe interactions in the rhizosphere is important for degradation of insecticides. For example Sun et al. (2015)26 reported that a DDT-degrading microbial community structure depended on plants growth.
Overall 80-90 percent of the soil processes are mediated by microorganisms27 and the transformation or degradation of pesticides into simpler molecules is dominated by several microbial processes including biodegradation and co-metabolism28. Biodegradation is the term used for the enzymatic process that utilises the insecticide as carbon/energy source - mineralising it into CO229. This type of microbial metabolism is considered to be the holy grail of biodegradation as it would theoretically thrive on the compound using it as energy and leave nothing but CO2. Co-metabolism is another type of degradation, where the pesticide is not the carbon-/energy source but is still degraded. In fact most of the breakdown of pesticides by microbes involves co-metabolism - where another substrate is metabolised with help of enzymes of mostly broad specificity - which by chance can also degrade a pesticide29.
Furthermore, transformation can also be catalysed chemically by soil and ‘humic’ particles. Clay minerals and metal oxides often dominate the clay-size fraction in soils and they are very reactive with any other molecule that possess a permanent or an induced charge - or in the case of oxides can even interact with non-ionic species30. So overall this highlights that the processes that can impact on dieldrin in soils are complex. And as said earlier, these processes are affected by abiotic and biotic factors which can change where, how and how long dieldrin is transformed. In the following paragraphs the available literature on these factors are reviewed.
Factors affecting the process of transformation of dieldrin
Climatic factors
Temperature and moisture effects all chemical and biological transformation processes and it is generally accepted that chlorinated hydrocarbons break down faster at higher soil temperatures and moisture content3132. The half-life of dieldrin was reported with large variations which is thought to be due to the differing climates of each of the experiments. It was reported as >20 years in a field trial on a light sandy soil in the Netherlands33; 7 years on a sandy loam under controlled conditions in Beltsville Maryland, US34; 7.5 months in subtrobical Taiwan35 and 117 days on a sandy loam in Haryana, India36. The half-life of dieldrin was also 3-5 times shorter under constant 30˚C compared to outdoor conditions as reported by Ghadiri et al. (1995)37 in Brisbane, Australia. However, climatic factors cannot be manipulated by property managers hence other soil and/or plant factors should be reviewed to discuss their potential for managing dieldrin remediation.
Soil texture and organic matter
The clay content of soils strongly affects soil’s ability to transform insecticides21. However, there are no known transformation effects of clay on dieldrin apart from adsorption. Although soil texture does affect the initial penetration of dieldrin into the soil profile - penetrating deeper in moist sandy soils compared to soils higher in silt and clays as reported after a soil column experiment which tested seven different soils38.
Other reports stated that soil organic matter (SOM) dominates the adsorption and transformation of pesticides 3940 with the hypothesis that its numerous functional groups and its higher reducing capacity increase the rate of chemical transformation of pesticides41. Soil organic C content for example was an important factor for sequestration of the organochlorine DDT42. Krishna and Phillip (2008)43 further reported that the general order of sequestration potential of organochlorines of different types of soils was compost soils > clayey soil > red soil > sandy soil, indicating that organic matter played the largest role in sorption processes. The hydrophobic fraction of SOM appeared particularly important for the sorption or transformation of nonionic molecules such as dieldrin. Notably Khan (1978)44 cited the enhancement of dehydrochlorination of chlorinated hydrocarbons such as DDT and lindane by SOM. Furthermore, reducing soils produced soluble species of ferrous iron (Fe2+) or sulfur (S) 45 that can potentially be involved in catalysing the degradation of insecticides such as dieldrin. It follows that organic soil amendments such as manures or flooding of a paddock can reduce the soil redox potential and therefore enable an increased rate of abiotic reductive processes although this is more likely to occur in deeper soil layers30.
Soil pH
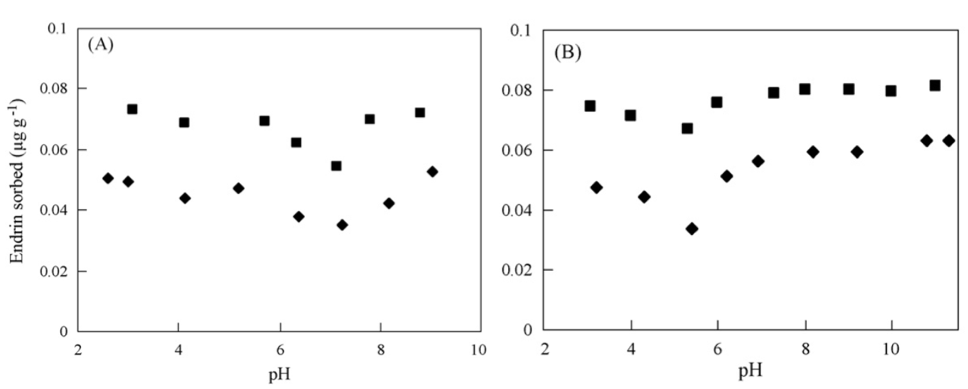
Fumio (1985)5 stated that overall chlorinated hydrocarbons persist longer in acidic soils compared to alkaline soils. Chapman and Cole (1982)46 tested the effect of pH (pH 4.5, 5, 6, 7 and 8) on heptachlor (another cyclodiene compound) in sterile water-ethanol and found that its half-life was 1.8-fold greater at pH 4.5 relative to pH 8. However, this was done in a liquid phase, and in the same study this correlation was not observed in mineral soils. It is likely that pH-dependent soil colloids, either from the SOM or the metal oxide fraction, play a role in the effectiveness of abiotic degradation. Wolfe et al. (1990)30 cited that amorphous metal oxides for example are likely to coat clay minerals and effectively negating their negative charge and therefore reactivity. And this negating effect would be more pronounced at low pH because it would increase availability of the oxides. Despite the low to zero charge of dieldrin, there was some evidence of its reactivity with pH-depended clay surfaces in soils. It was reported that the sorption of endrin (a stereoisomer of dieldrin) to kaolinites and montmorillonite decreased towards the zero point of charge and increased as either the pH decreases (i.e. more positive charges) or increased (i.e. more negative charges) from that point47. Based on these results, it may be possible that dieldrin is transformed more easily at a certain pH depending on soil mineralogy - if it behaves like its stereoisomer endrin. Lastly, soil pH markedly affects microbial activity and therefore biodegradation of contaminants such as dieldrin4849.
Light
Dieldrin is known to be photolysed by UV radiation in the topsoil or leaf surfaces into a more polar molecule named photodieldrin, with a similar level of toxicity to dieldrin505152. Photodieldrin molecules are chiral and exist as pairs of enantiomers and existed in soils even 40 years after application of dieldrin53. Given its toxicity and potential effect on soil photodieldrin should be considered when assessing soils for dieldrin contamination.
Plant species
The uptake of dieldrin by plants is also determined by the physiological traits of individual plant species. In the tropics, the castor bean crop (Ricinus communis L.) is effective at taking up POPs such as dieldrin and was therefore proposed for phyto-extraction remediation54. Furthermore, plants in the gourd family (Cucurbitaceae) including pumpkins, zucchinis, cucumbers and melons have shown to take up more dieldrin than all other plants considered in the study by Otani et al. (2007)25. The processes that lead to their uptake could be further investigated as a potential mechanism to desorb dieldrin to make it available for microbial degradation. Moreover, plants and their interaction with microbes in the rhizosphere may result in increased rates of microbial degradation processes. Sun et al. (2015)26 for example showed that degradation effects on DDT in mesocosms with a heavily contaminated soil from Wuhan, China, was highest when Orychophragmus violaceus was grown with adjusted soil nutrients.
Microbial communities
As mentioned, microbes can metabolise insecticides. While microbial degradation was observed for other chlorinated hydrocarbons such as DDT-analogues or benzene hexachlorides it was found that not many microorganisms were able to transform dieldrin probably because the chlorine-containing ring is particularly stable (Fumio 1985)5. The first evidence of biodegradation of dieldrin was reported by Matsumura and Boush (1967)55 who showed that 10 cultures (out of 500 cultured) were indeed active in degrading dieldrin compounds producing water-soluble and solvent-soluble metabolites. The active microbes were found to be the fungus Trichoderma viride and bacteria in the genus Pseudomonas and Bacillus. One year later, the same team isolated and identified the main metabolites and reported five metabolites but the major terminal compound appeared to be 6-7-trans-dihydroxydihydroaldrin (aldrin-diol) which is less toxic than dieldrin55. Wedemeyer (1968)56 further discovered that dieldrin was hydrolysed aerobically by Aerobacter aerogenes into aldrin trans-diol. Later, Wedemeyer (1968)56 found that the soil fungus Trichoderma koningii was able to thrive on dieldrin as its only carbon source, evidently metabolising the carbon as shown through the measurement of CO2 respiration of C14-labeled dieldrin57. More discoveries of dieldrin-degrading fungi followed recently: Cordyceps militaris KS-92 and C. brongniartii ATCC66779 with aldrin-diol and dihidrochlorodenedicarboxylic acid as metabolites58 and strain YK543 which is closely related to Phlebia brevispora Nakasone TMIC33929 with the metabolite 9-hydroxydieldrin which was discovered for the first time58.
The most promising strain was discovered in vitro by Kataoka (2010)59 that is more effective than any prior reported microorganisms. They identified the fungus Mucor racemosus strain DDF (Genbank accession number AY213659) that could degrade 95.8% of dieldrin into the main metabolite aldrin trans-diol. The degradation experiment was done in liquid medium using 35 strains of Trichoderma sp. and 36 strains of soil isolates (from agricultural soils that received frequent endosulfane treatment in Kagoshima, Japan), combined with DNA extraction and sequence analysis. This strain was effective across a pH range of 4-6 but less effective when nitrogen and carbon (glucose) sources are decreased in the growth medium which would indicate co-metabolism and not catabolism. It was also able to degrade DDT, DDE, heptachlor, heptachlor epoxide and endosulfan hence could be useful for a wide range of soils and situations. Prior to this a maximum dieldrin degradation of only 30% was reported so this is overall promising. However, this strain can probably only be isolated from this particular endosulfane-treated soil in Kagoshima, Japan. And to date not much is known about the metabolic processes leading to the conversion of dieldrin to aldrin trans-diol which could just be an intermediate product.
Despite these encouraging results, not one inoculum incorporated into soil was reported to be effective to remediate soils from dieldrin contamination, probably due to environmental conditions that are necessary for the survival of microbial strains60 or their inter-dependence to other soil microbial communities61. Furthermore, investigations on the degradation of other types of hydrocarbons such as diesel suggests that different soils have “different inherent microbial potential”62. Berthold (2016)62 also proposes that fungal mycelia promote horizontal gene transfer among bacteria reaffirming that a whole microbial community view may be necessary. This indicated that the relationships or dependencies between different organisms, or the requirement of key species to emerge from a whole microbial community, can be more important to metabolise dieldrin than just a single dieldrin degrading strain.
Conclusion and potential for bioremediation of dieldrin
Dieldrin, together with DDT, is one of the most persistent organic insecticides known to man, and despite attempts to understand degradation mechanisms there are wide knowledge gaps. Several factors determine the rate of degradation of dieldrin and large differences in its half-life have been observed. The single biggest factor appears to be climate, i.e. temperature and rainfall but these cannot be managed by property owners. However, SOM concentrations, texture, soil amendments and type of vegetation or crop all can affect removal, retention and transformation processes such as adsorption, volatilisation, photodecomposition, the erosional removal, leaching into the lower soil profile and chemical- and microbial transformation. Not much is known about the toxicity, bio-selectivity and uptake of the metabolic products of dieldrin degradation such as the enantiomers of photodieldrin, aldrin trans-diol and 9-hydroxydieldrin. Furthermore, it is unclear how plants may affect microbial communities to increase dieldrin degradation in the rhizosphere and if this can be optimised with nutrient management. Nevertheless, eighty to ninety percent of soil processes are mediated by microorganisms and recent advances in bioremediation by microorganisms capable of degrading hazardous organics are most appealing because they are affordable and can potentially metabolise contaminants into their non-toxic inorganic constituents. Hence, future research in this area may aim to find ways to utilise dieldrin degrading microorganisms to speed up the rate of dieldrin degradation and therefore remediate topsoils in a time-frame that is practical for current generations of land owners. However, a universal microbial bioremediation approach appears to be unrealistic, given the heterogeneity and interdependence of soils properties and microbial communities.
References
Lallas PL (2001) The Stockholm Convention on persistent organic pollutants. American Journal of International Law 692-708. ↩︎
NORM Program, 1996. Manual of procedures. Australian Government, Victoria. ↩︎
Corrigan P, Seneviratna P (1990) Occurrence of organochlorine residues in Australian meat. Australian Veterinary Journal 67, 56-58. ↩︎
Matsumoto E, Kawanaka Y, Yun S-J, Oyaizu H (2009) Bioremediation of the organochlorine pesticides, dieldrin and endrin, and their occurrence in the environment. Applied Microbiology and Biotechnology 84, 205-216. ↩︎
Fumio M (1985) ‘Toxicology of insecticides.’ (Springer US: New York) ↩︎
Gunther F, Westlake W, Jaglan P (1968) Reported solubilities of 738 pesticide chemicals in water. In ‘Residue Reviews.’ pp. 1-148. (Springer: New York) ↩︎
Mercier M (2013) ‘Criteria (dose/effect Relationships) for Organochlorine Pesticides: Report of a Working Group of Experts Prepared for the Commission of the European Communities, Directorate-General for Employment and Social Affairs, Health and Safety Directorate.’ (Elsevier: Brussels, Belgium) ↩︎
Zweig G (2013) ‘Principles, Methods, and General Applications: Analytical Methods for Pesticides, Plant Growth Regulators, and Food Additives.’ (Elsevier: Syracuse, N.Y. 13210) ↩︎
Gouin T, Mackay D, Jones KC, Harner T, Meijer SN (2004) Evidence for the “grasshopper” effect and fractionation during long-range atmospheric transport of organic contaminants. Environmental Pollution 128, 139-148. ↩︎
Hung H, Halsall CJ, Blanchard P, Li H, Fellin P, Stern G, Rosenberg B (2002) Temporal trends of organochlorine pesticides in the Canadian Arctic atmosphere. Environmental Science & Technology 36, 862-868. ↩︎
El Beit IO, Wheelock JV, Cotton DE (1981) Factors affecting soil residues of dieldrin, endosulfan, γ-HCH, dimethoate, and pyrolan. Ecotoxicology and Environmental Safety 5, 135-160. ↩︎
Weaver TB, Ghadiri H, Hulugalle NR, Harden S (2012) Organochlorine pesticides in soil under irrigated cotton farming systems in Vertisols of the Namoi Valley, north-western New South Wales, Australia. Chemosphere 88, 336-343. ↩︎
Martijn A, Bakker H, Schreuder R (1993) Soil persistence of DDT, dieldrin, and lindane over a long period. Bulletin of Environmental Contamination and Toxicology 51, 178-184. ↩︎
Rittmann BE (1994) ‘In situ bioremediation.’ (Taylor & Francis: Park Ridge, N.J., U.S.A) ↩︎
Zhang Wx, Bouwer EJ, Ball WP (1998) Bioavailability of Hydrophobic Organic Contaminants: Effects and Implications of Sorption‐Related Mass Transfer on Bioremediation. Groundwater Monitoring & Remediation 18, 126-138. ↩︎
Matsumura F (2012) ‘Biodegradation of pesticides.’ (Springer Science & Business Media: New York) ↩︎
Huang J-C, Liao L (1970) Adsorption of pesticides by clay minerals. Journal of the American Society of Civil Engineers 96, 1057-1076. ↩︎
Pignatello JJ, Xing B (1995) Mechanisms of slow sorption of organic chemicals to natural particles. Environmental Science & Technology 30, 1-11. ↩︎
Koskinen WC, Harper SS (1990) The retention process: mechanisms. Pesticides in the Soil Environment: Processes, Impacts, and Modeling. 51-77. ↩︎
Mills A, Biggar J (1969) Solubility-temperature effect on the adsorption of gamma-and beta-BHC from aqueous and hexane solutions by soil materials. Soil Science Society of America Journal 33, 210-216. ↩︎
Green RE (1974) Pesticide-clay-water interactions. Pesticides in Soil and Water 3-37. ↩︎
Morrison DE, Robertson BK, Alexander M (2000) Bioavailability to earthworms of aged DDT, DDE, DDD, and dieldrin in soil. Environmental Science & Technology 34, 709-713. ↩︎
Sicbaldi F, Sacchi GA, Trevisan M, DelRe AAM (1997) Root uptake and xylem translocation of pesticides from different chemical classes. Pesticide Science 50, 111-119. ↩︎
White JC, Mattina MI, Lee W-Y, Eitzer BD, Iannucci-Berger W (2003) Role of organic acids in enhancing the desorption and uptake of weathered p, p′-DDE by Cucurbita pepo. Environmental Pollution 124, 71-80. ↩︎
Otani T, Seike N, Sakata Y (2007) Differential uptake of dieldrin and endrin from soil by several plant families and Cucurbita genera. Soil science and plant nutrition 53, 86-94. ↩︎
Sun G, Zhang X, Hu Q, Zhang H, Zhang D, Li G (2015) Biodegradation of dichlorodiphenyltrichloroethanes (DDTs) and hexachlorocyclohexanes (HCHs) with plant and nutrients and their effects on the microbial ecological kinetics. Microbial Ecology 69, 281-292. ↩︎
Nannipieri P, Badalucco L (2003) Biologieal Processes. Handbook of Processes and Modeling in the Soil-Plant System 57. ↩︎
Bollag J, Liu S (1990) Biological transformation processes of pesticides. Pesticides in the Soil Environment: Processes, Impacts, and Modeling. 169-211. ↩︎
Cheng H-H (1990) ‘Pesticides in the soil environment: Processes, impacts, and modeling.’ (Soil Science Society of America: Madison, WI 53711 USA) ↩︎
Wolfe NL, Mingelgrin U, Miller GC (1990) Abiotic transformations in water, sediments, and soil. Pesticides in the Soil Environment: Processes, Impacts, and Modeling. 103-168. ↩︎
Lichtenstein EP, Schulz K (1959) Persistence of some chlorinated hydrocarbon insecticides as influenced by soil types, rate of application and temperature. Journal of Economic Entomology 52, 124-131. ↩︎
Komprda Jí, Komprdová Kr, Sáňka M, Možný M, Nizzetto L (2013) Influence of climate and land use change on spatially resolved volatilization of persistent organic pollutants (POPs) from background soils. Environmental science and technology 47, 7052-7059. ↩︎
Martijn A, Bakker H, Schreuder R (1993) Soil persistence of DDT, dieldrin, and lindane over a long period. Bulletin of Environmental Contamination and Toxicology 51, 178-184. ↩︎
Nash RG, Woolson EA (1967) Persistence of chlorinated hydrocarbon insecticides in soils. Science 157, 924-927. ↩︎
Talekar NS, Sun L-T, Lee E-M, Chen J-S (1977) Persistence of some insecticides in subtropical soil. Journal of Agricultural and Food Chemistry 25, 348-352. ↩︎
Singh G, Kathpal T, Spencer W, Dhankar J (1991) Dissipation of some organochlorine insecticides in cropped and uncropped soil. Environmental Pollution 70, 219-239. ↩︎
Ghadiri H, Rose C, Connell D (1995) Degradation of organochlorine pesticides in soils under controlled environment and outdoor conditions. Journal of Environmental Management 43, 141-151. ↩︎
Carter FL, Stringer CA (1970) Soil moisture and soil type influence initial penetration by organochlorine insecticides. Bulletin of Environmental Contamination and Toxicology 5, 422-428. ↩︎
Burns RG (1975) Factors affecting pesticide loss from soil. Soil Biochemistry 103-141. ↩︎
Hamaker JW (1975) The interpretation of soil leaching experiments. In ‘Environmental dynamics of pesticides.’ pp. 115-133. (Springer: New York) ↩︎
Stevenson FJ (1982) ‘Humus chemistry.’ (Wiley&Sons, NY: New York) ↩︎
Zhang N, Yang Y, Tao S, Liu Y, Shi K-L (2011) Sequestration of organochlorine pesticides in soils of distinct organic carbon content. Environmental Pollution 159, 700-705. ↩︎
Krishna KR, Philip L (2008) Adsorption and desorption characteristics of lindane, carbofuran and methyl parathion on various Indian soils. Journal of Hazardous Materials 160, 559-567. ↩︎
Khan S (1978) The interaction of organic matter with pesticides. Developments in Soil Science 8, 137-171. ↩︎
Wahid P, Sethunathan N (1979) Involvement of hydrogen sulphide in the degradation of parathion in flooded acid sulphate soil. Nature 282, 401-402. ↩︎
Chapman R, Cole C (1982) Observations on the influence of water and soil pH on the persistence of insecticides. Journal of Environmental Science and Health Part B 17, 487-504. ↩︎
Peng X, Wang J, Fan B, Luan Z (2009) Sorption of endrin to montmorillonite and kaolinite clays. Journal of Hazardous Materials 168, 210-214. ↩︎
Alexander M (1999) ‘Biodegradation and bioremediation.’ (Academic Press: Cambridge, Massachusetts) ↩︎
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. The ISME journal 4, 1340. ↩︎
Robinson J, Richardson A, Bush B, Elgar K (1966) A photoisomerisation product of dieldrin. In ‘Bulletin of Environmental Contamination and Toxicology.’ pp. 127-132. (Springer: Sittingbourne, Kent, U.K) ↩︎
Henderson GL, Crosby DG (1968) The photodecomposition of dieldrin residues in water. Bulletin of Environmental Contamination and Toxicology 3, 131-134. ↩︎
Walton MS, Beck BV, Baron RL (1971) Subchronic toxicity of photodieldrin, a photodecomposition product of dieldrin. Toxicology and Applied Pharmacology 20, 82-88. ↩︎
Buser H-R, Müller MD, Buerge IJ, Poiger T (2009) Composition of aldrin, dieldrin, and photodieldrin enantiomers in technical and environmental samples. Journal of Agricultural and Food Chemistry 57, 7445-7452 ↩︎
Rissato SR, Galhiane MS, Fernandes JR, Gerenutti M, Gomes HM, Ribeiro R, de Almeida MV (2015) Evaluation of Ricinus communis L. for the Phytoremediation of Polluted Soil with Organochlorine Pesticides. Biomed Research International ↩︎
Matsumura F, Boush G (1967) Dieldrin: degradation by soil microorganisms. Science 156, 959-961. ↩︎
Wedemeyer G (1968) Partial hydrolysis of dieldrin by aerobacter aerogenes. Applied Microbiology 16, 661. ↩︎
Bixby M, Boush G, Matsumura F (1971) Degradation of dieldrin to carbon dioxide by a soil fungusTrichoderma koningi. Bulletin of Environmental Contamination and Toxicology 6, 491-494. ↩︎
Xiao PF, Kondo R (2013) Biodegradation of dieldrin by cordyceps fungi and detection of metabolites. Applied Mechanics and Materials 295, 30-34. ↩︎
Kataoka R, Takagi K, Kamei I, Kiyota H, Sato Y (2010) Biodegradation of Dieldrin by a Soil Fungus Isolated from a Soil with Annual Endosulfan Applications. Environmental Science & Technology 44, 6343-6349. ↩︎
Anderson J, Lichtenstein E, Whittingham W (1970) Effect of Mucor alternans on the persistence of DDT and dieldrin in culture and in soil. Journal of Economic Entomology 63, 1595-1599. ↩︎
Berthold T, Centler F, Hübschmann T, Remer R, Thullner M, Harms H, Wick LY (2016) Mycelia as a focal point for horizontal gene transfer among soil bacteria. Scientific Reports 6, 36390. ↩︎
Bundy J, Paton G, Campbell C (2002) Microbial communities in different soil types do not converge after diesel contamination. Journal of Applied Microbiology 92, 276-288. ↩︎